Engineering
Melonizing
During Melonizing® a nitrocarburized layer is formed consisting of the outer compound layer (ε-iron nitride) and the diffusion layer thereunder. The formation, microstructure and properties of the compound layer are determined by the base material. The compound layer consists of compounds of iron, nitrogen, carbon and oxygen. Due to its microstructure, the compound layer does not possess metallic properties. It is particularly resistant to wear, seizure and corrosion, as well as being stable almost to the temperature at which it was formed. Compared with plasma or gas nitro-carburizing, compound layers with the highest nitrogen content can be obtained by Melonizing®. Layers with a high nitrogen content give better protection against wear, and in particular corrosion, than those with a low content.
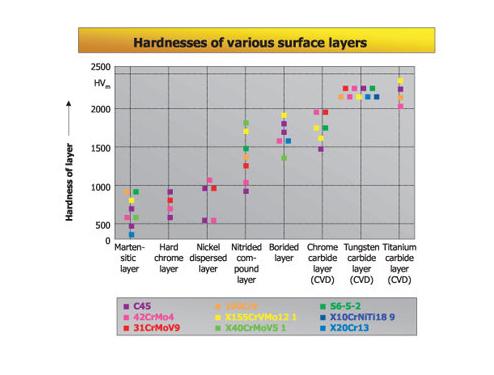
Hardness of Various Surface Layers
Depending on the material used, the compound layer will have a Vickers hardness of about 800 to 1500 HV. The graph above shows a comparison of the surface layers produced by various processes and their hardness. In the metallographic analysis of salt bath nitrocarburized components, that part of the total layer known as the compound layer is defined clearly from the diffusion layer thereunder as a slightly etched zone. During the diffusion of atomic nitrogen the compound layer is formed. The growing level of nitrogen results in the limit of solubility in the surface zone being exceeded, which causes the nitrides to precipitate and form a closed compound layer.
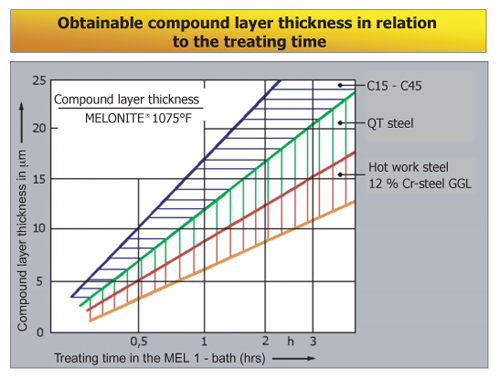
Obtainable Compound Layer Thickness
In addition to the treatment parameters (temperature, duration, bath composition), the levels of carbon and alloying elements in the materials to be treated influence the thickness of layer obtainable. Although the growth of the layer is lower the higher the content of alloy, the hardness however increases to an equal extent.
The data shown in the graph above were determined in a MEL1 / TF1 bath at 1076°F. With the usual treating durations of 60-120 minutes, the compound layer obtained was 10-20 μm thick on most qualities of material.






