Understanding the Diffusion Layer
Nitriding Depth & Hardness
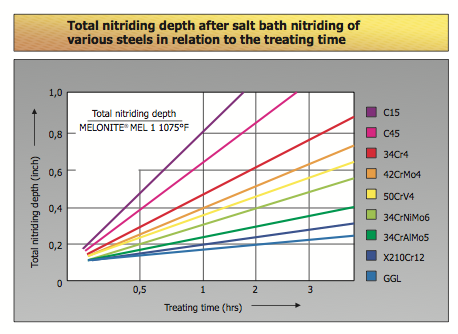
The depth and hardness of the diffusion layer are largely determined by the material. The higher the alloying content in the steel, the lower the nitrogen penetration depth at equal treating duration. On the other hand, the hardness increases the higher the alloying content. In the case of unalloyed steels, the crystalline structure of the diffusion. In the metallographic analysis of salt bath nitrocarburized components, that part of the total layer known as the compound layer is defined clearly from the diffusion layer there under as a slightly etched zone. During the diffusion of atomic nitrogen the compound layer is formed. The growing level of nitrogen results in the limit of solubility in the surface zone being exceeded, which causes the nitrides to precipitate and form a In addition to the treatment parameters (temperature, duration, bath composition), the levels of carbon and alloying elements in the materials to be treated influence the thickness of layer obtainable.
Total Nitriding Depth
Although the growth of the layer is lower the higher the content of alloy, the hardness however increases to an layer is influenced by the rate of cooling after nitrocarburizing. After rapid cooling in water, the diffused nitrogen remains in solution. If cooling is done slowly, or if a subsequent tempering is carried out, some of the nitrogen could precipitate into iron nitride needles in the outer region of the diffusion layer of un-alloyed steels. This precipitation improves the ductility of nitro-carburized components. Unlike unalloyed steels, part of the diffusion layer of high alloyed materials can be better identified metallographically from the core structure, due to the improved etchability. But the actual nitrogen penetration is also considerably deeper than the darker etched area visible metallographically. Cooling does not influence the formation of the diffusion layer to any noteworthy extent.